Recently, transcranial ultrasound has been getting some press. This has been a deep interest for a long time so I thought I would write up where the clinical results are at present. I haven’t covered the important work which combines transcranial ultrasound with, for example, cancer drugs. This is only about mental illness and […]
Medical Devices
What level of experience is required for expert testimony?
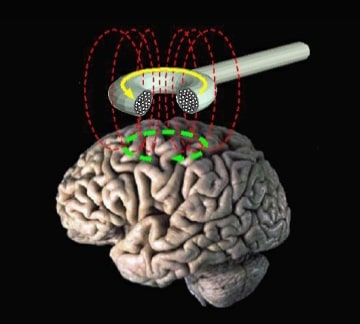
Transcranial Magnetic Stimulation (TMS) is a non-drug treatment for illnesses like depression which is gaining clinical adoption after FDA clearance of new protocols such as SAINT. Brain Frequency LLC and Wave Neuroscience Inc. both offer types of personalized TMS involving a pre-procedure electroencephalogram (EEG) assessment. In recent Western District of Texas patent litigation between these […]
The Cybersecurity Sword Hanging Over Medical Device Manufacturers
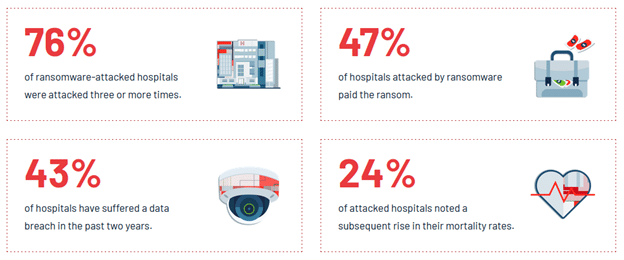
Introduction In September 2023, the U.S. Food and Drug Administration (FDA) issued a pivotal guidance document titled Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions. This guidance aims to bolster the cybersecurity of medical devices, thus safeguarding patients, healthcare facilities, and the broader healthcare ecosystem from cyber threats. The new regulations […]
Medical device security expert witness: improving critical products
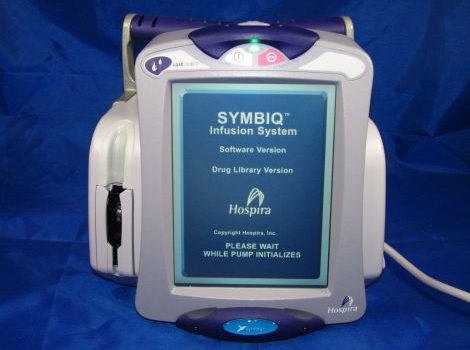
Medical devices — engineering inventions to improve human health — have more than a century of history, with the global medical device market size predicted to exceed $600 billion by 2025. Most products in this market share a common feature: network connectivity. Figure 1 shows a typical example: even something as mundane as a pump […]
Medical Device Software Standard of Care Expert Witness: Causes, Standards & Issues
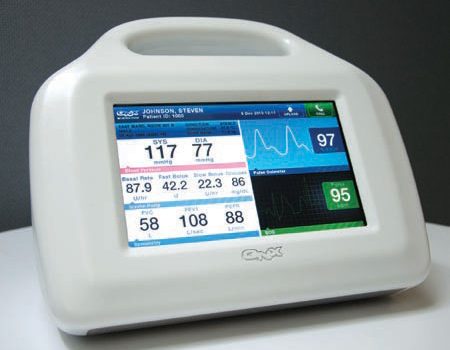
What Medical Device Software is What is medical device software? And why would its “standard of care” be an important subject? In simple terms, medical device software is a component of, or an accessory to, a piece of electronics that interacts with the human body (see figure 1). “Standard of Care” is a key phrase […]